-
What is the PH raising to ? Peat itself is acidic 4.0- 5.0 so the raising of your PH must have something to do with the input water you're using, or amendments like compost that have been added. Here's a look at what watering can do to PH.
Leaching washes out nutrients and raises pH can do to PH.
Fig. 2 shows an experiment by Angelica Cretu at University of Florida where she added increasing volumes of pure water to a peat/perlite growing medium. The medium had an initial nutrient pre-plant charge using water soluble fertilizer that raised EC to 2.1 mS/cm using the saturated paste extract method. By the time about one container volume of clear water was washed through the substrate, the nutrients were almost completely leached out and the EC was down to 0.4 mS/cm. As the substrate-EC declined, the substrate-pH increased. This effect also happens under mist propagation when excess water washes through the plug tray and pre-plant nutrients are leached out.
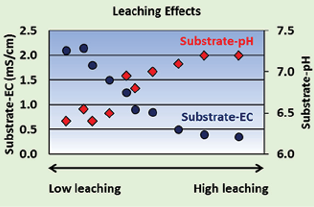 Fig. 2. Effects of leaching with clear water on substrate-pH and substrate-EC.
Fig. 2. Effects of leaching with clear water on substrate-pH and substrate-EC.Cation exchange capacity
Peat is naturally acidic, and lime is usually added to the substrate to bring pH to around 5.6 to 6.4. Lime is typically calcium and magnesium carbonate. The lime reaction adds base (carbonate) that neutralizes acid from the peat. Lime also supplies calcium and magnesium.
Calcium (Ca2+) and magnesium (Mg2+) are examples of nutrients that have a positive charge and are called “cations.” Acid (which is a proton, also written as H+) is also a cation.
The surface of peat particles has many negative charged locations called “cation exchange sites.” These negatively-charged sites attract positively charged ions, because the peat and cations have opposite electrical charges. The cations adsorbed to the peat include nutrients such as calcium and magnesium, and also protons. The “cation exchange capacity” or CEC refers to the amount of cations that can be held at these exchange sites by a certain weight of substrate. “Base saturation” refers to the percentage of exchange sites occupied by cations such as calcium and magnesium, rather than protons.
Adding lime before planting tends to saturate most cation exchange sites with calcium and magnesium, displace the acid on the peat, and neutralizes the acid with carbonate. Nutrients are also added to the substrate from other sources, including fertilizers and irrigation water.
Adding calcium and magnesium displaces acid protons (H+) from the unsaturated exchange sites. These displaced protons move into the soil solution and drop pH (Fig. 3A). In contrast, leaching with clear water removes cations such as calcium and magnesium from the substrate exchange sites and soil solution. Protons in the soil solution then adsorb back onto the peat, which raises pH of the soil solution (Fig. 3B).
0 0 0 0 0 0 0
Comment to Soil ph keep increase
